More wavelengths—better insights
In biophotonics, the trend is moving towards multimodal imaging and platforms which uncover the secrets of cells with variable wavelengths and procedures.
Where does the tumor end and where does the healthy tissue start? In order to answer this question during surgery, the EU research project CARMEN involving the Laser Center Hanover (LZH) and the Belgian innovation center MULTITEL is developing a new multimodal imaging system. At the heart of the system is a tunable, fiber-based ultra-short pulse beam source which gives off either femto or picosecond pulses in various light wavelengths. The compact device allows tissue analyses using anti-Stokes Raman spectroscopy (CARS), multiphoton microscopy or STED (Stimulated Emission Depletion) microscopy. Operating teams could therefore analyze biopsies with all three procedures and combine them in order to identify the cells contained precisely. According to LZH, multimodal imaging is also suitable for tracking drugs and nanoparticles in cells and tissues and for microscopically checking the effectiveness of cosmetic products.
The platforms are coming
With their approach, LZH and MULTITEL are following the trend. On the basis of miniaturized hardware, progressing digitalization and automation, biophotonics providers are offering medical and scientific researchers increasingly differentiated insights into cells, tissue and biochemical processes. With the SYIONS platform, JENOPTIK too is driving this development. Thanks to configurable modules for the light source, optical system, electronics, sensors and software, this can be tailored specifically to diagnostic or scientific questions. At the same time, various optical units can be combined in order to analyze samples in parallel and in a spectrally differentiated manner or to use more colors and varying magnification levels in imaging. In order to achieve higher imaging rates and differentiated recordings, a number of color and monochrome cameras can be integrated.
This flexibility allows more differentiated findings to be obtained from samples using multispectral analysis. And this is necessary to ensure that the hardware can keep up with the development of new fluorescence dyes. Because the dyes available range from UV to well into invisible IR wavelengths, light sources for stimulation and sensors for detecting the wavelengths emitted are needed. After all, this will enable teams of researchers to take advantage of the full potential for tracking the movement of various fluorescence-marked molecules on a cell level and processes running in parallel in living cells. In short: they will be able to decode previously hidden functions and mechanisms. Because signals of intensity, lifespan, concentration or absorption are often sufficient for this, the platform measures signals which are then depicted either as an image, a thermal image chart, a sequence or as a recognized molecule.
A new era of microscopy
In the blog for the SYIONS platform, people are talking about a new era of microscopy: after the breakthrough of electron microscopy, the introduction of antibody fluorescence staining, the increasing procedure differentiation and the breakthrough of software-assisted image and signal analyses, an era of integrated, multimodal platforms is dawning.
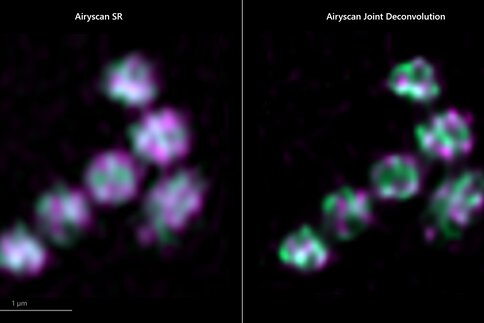
Modern microscopes are no longer used simply for observation purposes—they are precision measuring instruments too. Super resolution microscopes such as the ZEISS Elyra 7 allow structures just 60 nanometers apart to be distinguished in living samples. In conjunction with structured lighting, image reconstruction algorithms ensure that the dynamics as well as the resolution are right. This is the only way to observe biological processes in living cells or organisms: at resolutions below 100 nanometers, the microscope records up to 255 images per second. For laser scanning microscopes, Zeiss also offers the new Airyscan Joint Deconvolution: an area detector with 32 circularly arranged detection elements. Each of these acts as a small pinhole with a different view of the sample, providing spatial information.
The range of applications for these high-end microscopes is growing. They can be used for anything from cell imaging and detection or microbial diagnostics to microtumor analyses or detecting circulating tumor cell DNA. The latter opens up new opportunities for diagnosing cancer early on. After all, if the DNA of tumor cells can be detected in blood, a blood sample is sufficient to make an accurate early diagnosis. Quick and accurate blood tests can also be carried out using multi-parameter flow cytometry. This analyzes the composition of cells in blood flowing through a measuring zone which is irradiated by lasers in various wavelengths. Using fluorescence and scatter signals which photomultipliers or avalanche photodiodes measure, the cells contained in the sample can be identified. Five lasers with different wavelengths can measure over 25 different parameters. The first platforms work with ten lasers from ultraviolet to infrared. Coherent offers the CellX platform for this—it integrates diode-pumped solid-state lasers with a wide range of wavelengths.
More accurate diagnostics and personalized medicine
In addition to clinical diagnostics, the most important applications for multi-parameter flow cytometry are in immunology and drug research. The direction is clear: the aim is to refine diagnostics so that diseases can be diagnosed early on and treated with personalized, closely monitored treatments. Accurate blood analytics is a step in this direction. But light can do more. Photonics is allowing an increasingly detailed understanding of organ functions and processes in neural networks on a molecular level. For example, optogenetics follows brain activities using infiltrating protein molecules which can be switched using an ultrashort pulse laser. And this can be done so precisely that the interaction of hundreds of specifically controlled neurons can be seen and understood.
At the Fraunhofer Institute for Laser Technology (ILT), teams of researchers use laser procedures to identify particularly productive cell lines for obtaining biologicals. Biologicals are very much in demand in cancer therapy and for treating auto-immune diseases and rheumatic disorders. They are usually based on cell constituents or proteins produced by cells. Finding a suitable cell line for this is a complex, costly process. According to ILT, the costs of the process which can often take 12 months can be as much as €400 million. With the so-called OptisCell procedure, the institute is developing an approach which, thanks to a combination of various photonic processes, takes just three months. With marker-free Raman spectroscopy, it is possible to identify suitable, highly productive cell candidates which they then isolate using the LIFT (Laser Induced Forward Transfer) procedure. With the help of machine learning, this largely automated photonic process chain can quickly screen large number of cell lines. Using the specters, Raman spectroscopy recognizes which cells produce particularly large quantities of the required protein. These are then isolated with LIFT in a small steam bubble generated by a laser pulse. After that, the researchers use a third photonic process, surface enhanced Raman scattering (SERS) spectroscopy, to check whether the cells really are high-producer cells. Here too, an integrated platform combining UV and MIR beam sources with precision optical hardware is used.
The optics matters too
The precision analyses require not only the use of various laser wavelengths and sufficiently sensitive sensors. In order to detect the emissions of fluorescence molecules which are often weak, highly sensitive optical filters with maximum transmission are needed as well. Various firms including AHF analysentechnik AG specialize in the development of these filters for biophotonic applications. Without high-end optical systems, multiphoton microscopy where even non-fluorescent molecules are stimulated with a number of photons and then emit a photon with greater energy would not be possible. And without multiphoton beam splitters and suitable optical filters, it would not be possible to achieve the resolution which is possible today without damaging the biological samples—in spite of the power density of the ultrashort pulse lasers used.
